ข้อสอบ BMAT Part2 พร้อมเฉลย มาทดสอบกันพร้อมสอบแค่ไหน?
BMAT Part2
| A element | B element | C element |
| 2 8 | 2 8 2 | 2 8 7 |
- Element A is low reactive because of high stability.
- The ionization of element A is higher than element C.
- The oxide compound of element B has the empirical formula that is B2
- Element B is group 2A and period 3, and element C is group 5A and period 3.
- Element A is the gas state which is used for the fluorescence lamp.
Which statement is incorrect?
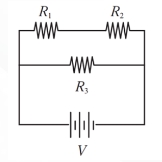
A is at (-6, 5), B is on the y-axis, and C is at point (0, -1).
AC is the diameter of the circle.
Line CQ is a segment of the line 5y = 3x - 5, and point O is on line BQ.
What is the value of 2b - a if the coordinates of Q are (a, b)?
Which one of these statements explains these changes?
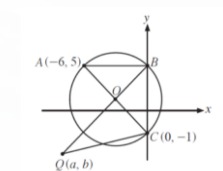
This process was discovered by Charles Goodyear in 1839 that Sulfur (S8) and ZnO as catalyst can be linked at double bonds between chains of natural rubber polymer.
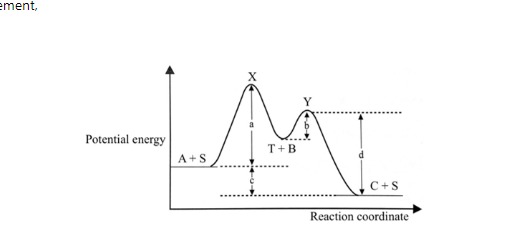
Following the figure.
11. An elevator is supported by a cable that connects to an electric motor. The elevator of mass of 783 kg is lifted vertically through a height of 22.0 m in a time of 180 s. If the motor is connected to a constant 110 V D.C. supply and the complete lifting system is 87% efficient.
What is the current in the electric motor? (Given gravitational field strength = 10 m⋅s–2)
Which row describes the processes correctly?
14. 8.28 grams of Lead metal sheet reacts with 1.2 M, 50 cm3 of Silver nitrate, in the Erlenmeyer flask is found Silver precipitate which has 3.24 grams and the reaction causes in the below.
Pb (s) + AgNO3 (aq) → Ag (s) + Pb(NO3)2 (aq)
What is the method to find the limiting reagent and how many percent yields of Silver precipitate? (Ar values: Pb = 207 ; Ag = 108 ; S = 32 ; O = 16)
15. An orange light ray with wavelength of 620 nm passes from air into liquid. Its speed decreases by 25%. What is the wavelength and the color of the light beam in the liquid?
The probability that the bus will be on time is 0.6.
The probability that the bus will be late is 0.4.
If the bus is on time, then the probability that he will catch the boat is 0.8.
If the bus is late, then the probability that he will catch the boat is 0.6.
Given that Tony catches the boat, what is the probability that the bus was on time?
17.The contents of the blood vary according to the current health and conditions of the body. Which one of these statements is NOT true about blood contents?
and following the statement
- If the catalyst is added into the reaction, the activation energy of this reaction will be lower than c.
- The energy of reaction should be A + B → C + c
- S is an intermediate compound and T is a catalyst.
- The stability of X and Y is higher than C.
- Increasing the temperature will decrease the activation energy of this reaction.
Which of the statements is true?
19.Which statement(s) below is(are) INCORRECT?
P) In a wood-fired pizza oven, the heat is transferred to the pizza only by convection.
Q) Using a curling iron to curl hair, the heat is transferred to the hair only by conduction.
R) If croissants are reheated in a microwave, most heat is transferred to the bread by radiation.
S) An object is floating in a vacuum chamber. When the rate of radiation emission is equal to the rate of radiation absorption; the object is at the same temperature as its surroundings.
Which one of the statements is NOT true regarding the generation of a mammalian embryo and the uses of embryonic stem cells?
The gases produced at the electrodes were collected and tested with a colorless aqueous solution of sodium iodide.
Which row in the following table best describes the observations in these tests?
|
| the pH of the remaining solution | test of gas from anode (positive electrode) | test of gas from cathode (negative electrode) |
| A. | 3 | no observable change | violet solid |
| B. | 3 | violet solid | no observable change |
| C. | 7 | no observable change | violet solid |
| D. | 7 | violet solid | no observable change |
| E. | 10 | no observable change | violet solid |
| F. | 10 | violet solid | no observable change |
Which statements are true regarding how living organisms and their environment changes over time?
- Deforestation decreases the amount of carbon dioxide converted by producers into biomass.
- An individual of a species evolves over time by natural selection as the environment changes.
- Recombinant DNA technology can contaminate natural population with undesirable genes leading to imbalances in food webs.
- The emergence of a disease can decrease the size of a population while increasing the size of another population living in the same habitat.
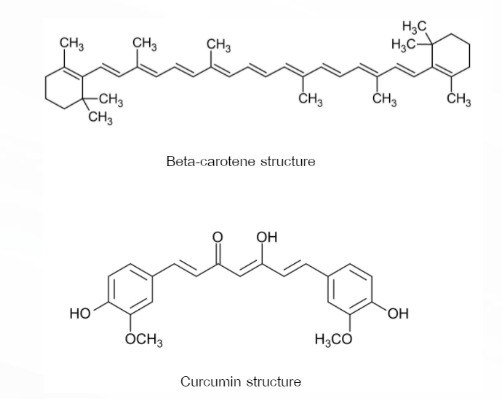
24. Beta-carotene is an orange-red natural dye that is extracted from many orange-red fruits and vegetables and Curcumin is a yellow natural dye extracted from Turmeric. Both compounds are natural dyes that do not react with each other and do not react with Methanol and Hexane.
Following the structure.
If the two natural dyes are mixed in the beaker which has the mixed solvent between Hexane and Methanol, what observation in the beaker is correct? (given density of C6H14 = 655 kg/dm3 ; density of CH3OH = 792 kg/dm3 and the mass of two natural dyes which is mixed in solvent does not change the density of solvents)
|
| The observation in the beaker |
| A. | The mixture is separated. The orange-red is the top of the beaker and the yellow is below the beaker. |
| B. | The mixture is separated. The yellow is the top of the beaker and the orange-red is below the beaker. |
| C. | The mixture is separated. The yellow is the top of the beaker and the clear solution is below the beaker. |
| D. | The mixture is separated. The clear solution is the top of the beaker and the yellow is below the beaker. |
| E. | The mixture is separated. The orange-red is the top of the beaker and the clear solution is below the beaker. |
| F. | The mixture is separated. The clear solution is the top of the beaker and the orange-red is below the beaker. |
| G. | The mixture becomes the orange solution. |
| H. | None of above |
25.Consider two uniform spherical planets of equal density but unequal radius.
Which of the following quantities will not change for both planets?
















