ข้อสอบ TBAT Chemistry พร้อมเฉลย สำหรับเตรียมความพร้อมก่อนสอบ
TBAT Chemistry
01. The diagram shows the result of dropping a purple crystal into water. Which processes take place in this experiment?
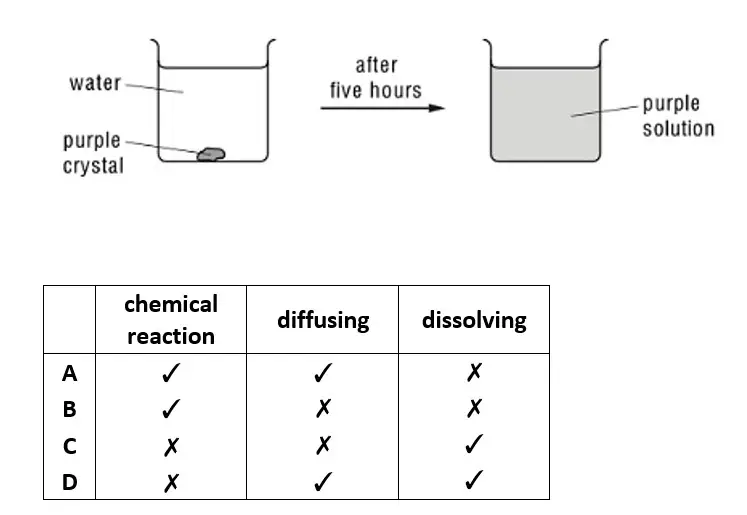
tbat chemistry q01
02. Which row about elements, mixtures and compounds is correct?
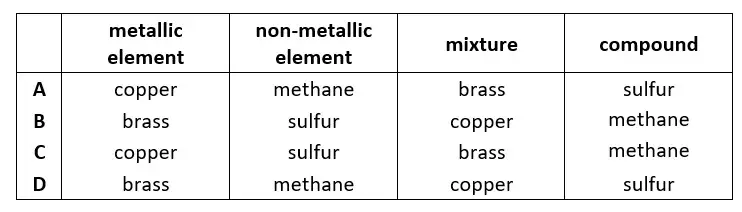
tbat chemistry q02
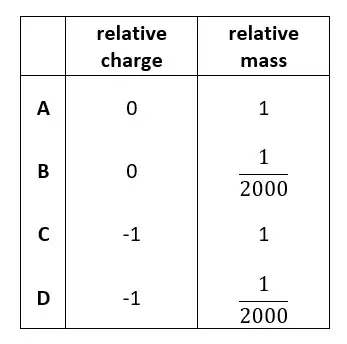
03. What are the relative charge and relative mass of an electron?
04. The atomic structures of four particles, W, X, Y and Z, are shown. Which particles are isotopes of the same element?
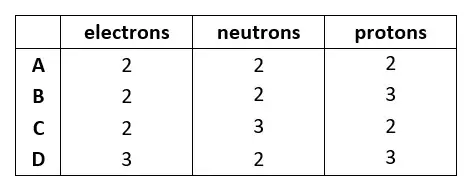
tbat chemistry q04
05. Which row shows the properties of an ionic compound ?
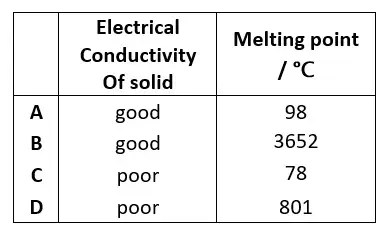
tbat chemistry q05
06. Which row describes the formation of single covalent bonds in methane?
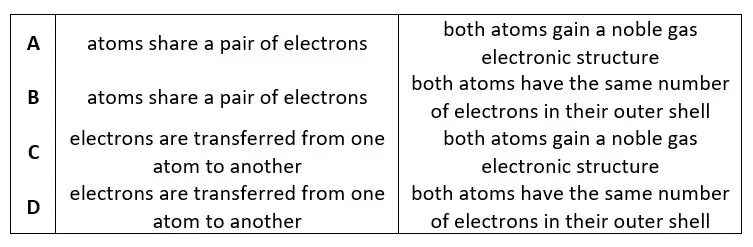
tbat chemistry q06
07. Which equation represents the neutralisation of nitric acid using sodium hydroxide?
08. What is the relative formula mass of ammonium nitrate, NH4NO3 ?
09. Concentrated aqueous sodium chloride is electrolysed using inert electrodes. Gases X and Y are produced at the electrodes shown.What are X and Y ?
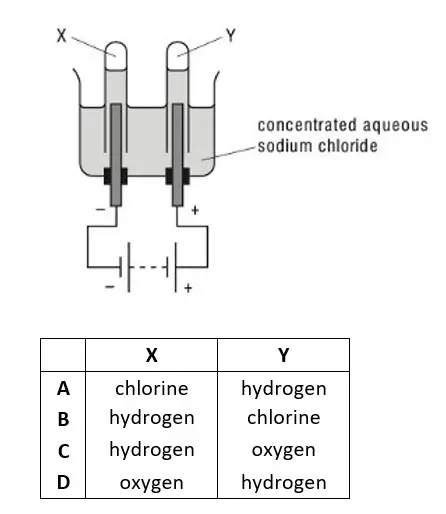
tbat chemistry q09
10. Which statement about hydrogen fuel cells is correct?
11. A reaction pathway diagram is shown.
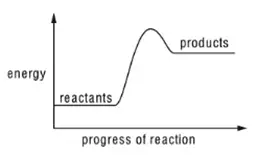
tbat chemistry q11
12. Lumps of calcium carbonate react with dilute hydrochloric acid as shown. CaCO3 + 2HC/ -> CaC/2 + H2O + CO2Which change in conditions decreases the rate of the reaction ?
13. Solid copper (II) sulfate exists in two different forms, anhydrous and hydrated.One of these forms is blue and the other is white.The change between these two forms is reversible.blue form ⇌ white formWhat is the blue form and how is the change from the blue form to the white form brought about ?

tbat chemistry ข้อ 13
14. Four redox equations and statements about the equations are shown. Which statements about the equations are correct?
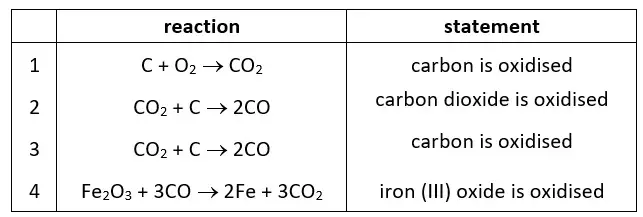
tbat chemistry q14
15. Sodium hydroxide forms an alkaline solution with a pH of 14. Which indicator turns yellow when added to this solution?
16. Which row identifies an acidic oxide and a basic oxide?
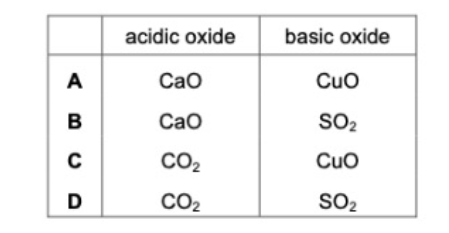
17. A Student makes aqueous copper (II) chloride by adding excess copper (II) carbonate to dilute hydrochloric acid. What is the next step in the method in the formation of solid copper (II) chloride ?
18. Which statements about the trends across a period of the Periodic Table are correct ?
- luminium is more metallic than sodium.
- Beryllium is more metallic than carbon.
- Boron is more metallic than lithium.
- Magnesium is more metallic than silicon.
19. Which row shows the trend in melting point, density and reactivity as Group is descended?
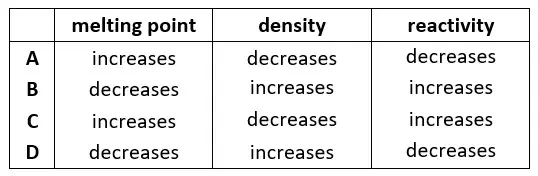
tbat chemistry q19
20. Which row describes a similarity and a difference between chlorine and bromine?
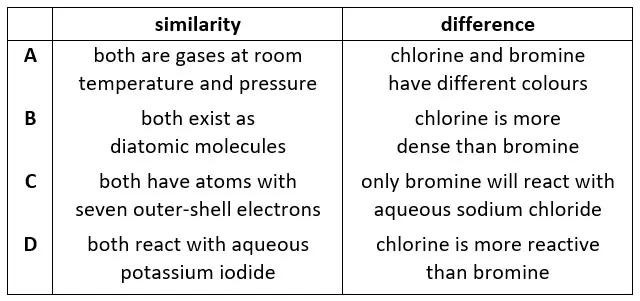
tbat chemistry q20
21. Which statement describes transition elements ?
22. Which diagram shows the electric structure of a noble gas ?
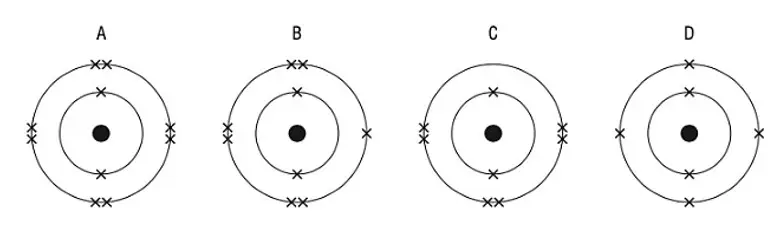
tbat chemistry q22
23. Which gas is made when powdered zinc is added to dilute hydrochloric acid ?
24. Which metal is used in aircraft manufacture because it has a low density ?
25. The diagram represents the structure of a solid. Which solids does the diagram represent ?
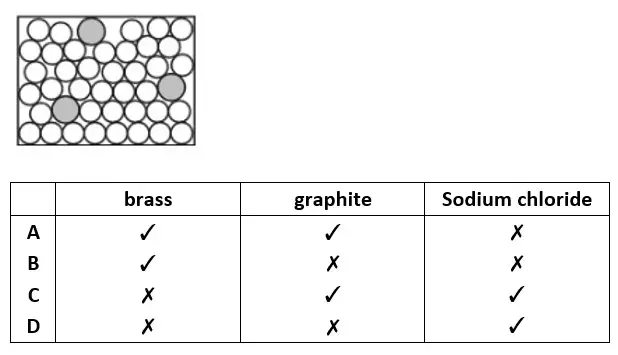
tbat chemistry q25
26. Three students, X, Y and Z, are told that solid P reacts with dilute acids and also conducts electricity. The table shows the students’ suggestions about the identity of P. Which students are correct ?
tbat chemistry q26
27. Which substances in the air are needed for iron to rust ?
28. Part of the reactivity series of metals is shown.
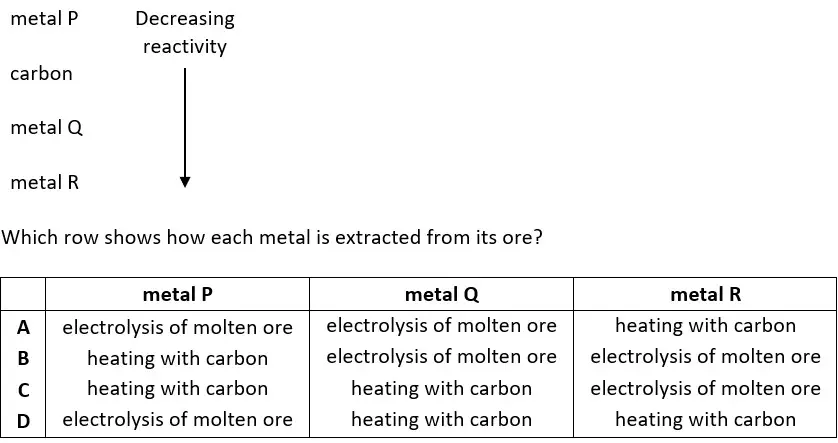
tbat chemistry q28
29. Which gas has the fastest rate of diffusion?
30. There are two stable isotopes of bromine. The mass number of isotope 1 is 79. The mass number of isotope 2 is 81. Which statement is correct ?
31. Which statement about ions and ionic bonds is correct ?
32. Part of the Periodic Table is shown. Which type of chemical bonding is present in the oxide of F and in the oxide of G?
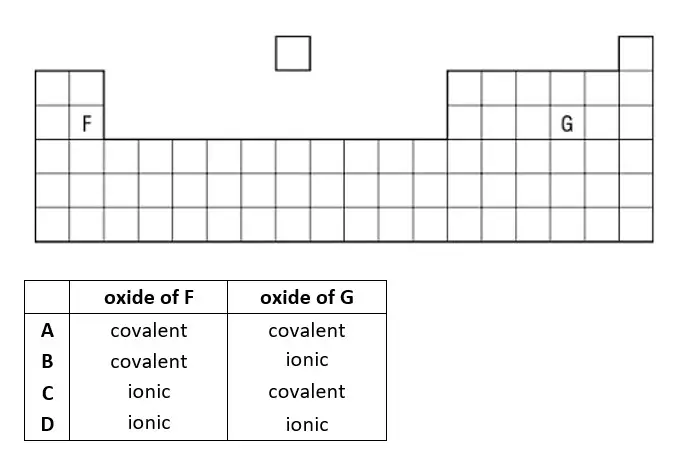
tbat chemistry q32
33. Elements X and Y react to form a compound. Element X loses two electrons and element Y gains one electron. What is the charge on the ions of elements X and Y and what is the formula of the compound ?
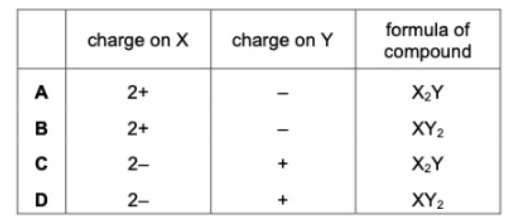
tbat chemistry ข้อ 33
34. Which statement about graphite explains why it is used as an electrode ?
35. Methane, CH4, burns in air to form carbon dioxide and water. What is the balanced equation for this reaction ?
36. The equation for the thermal decomposition of sodium hydrogen carbonate is shown.2NaHCO3 -> Na2CO3 + H2O + CO2The Mr of sodium hydrogen carbonate, NaHCO3, is 84.The Mr of sodium carbonate, Na2CO3, is 106.In an experiment, 2.1 g of sodium hydrogencarbonate is heated but not all of it decomposes. All of the carbon dioxide is collected and measured at room temperature and pressure. The total volume of carbon dioxide produced is 0.21 dm3.The volume of 1 mole of a gas at room temperature and pressure is 24 dm3.Which statement is correct ?
37. An electrolysis experiment is done using carbon electrodes.Hydrogen and oxygen are formed at the electrodes.What is the electrolyte ?
38. Concentrated aqueous copper(II) sulfate is electrolysed using copper electrodes.Which ionic half-equation describes the reaction taking place at the cathode ?
39. When powdered sodium carbonate and aqueous ethanoic acid are mixed, the temperature of the mixture falls.Which statement about this reaction is correct ?
40. Magnesium powder reacts with an excess of dilute hydrochloric acid to produce hydrogen gas.Which statement about this reaction are correct ?
- The smaller the particles of magnesium powder, the more slowly the hydrogen is produced.
- The higher the temperature, the faster the magnesium powder disappears.
- The lower the concentration of dilute hydrochloric acid, the faster the rate of reaction.
- The faster the magnesium powder disappears, the faster the rate of reaction.
41. The reaction between two aqueous compounds, X and Y, is slow and exothermic.The graph shows how the rate of this reaction changes with time.A student suggests that the rate of reaction decreases with time because:
- the activation energy decreases.
- the speed of the molecules of X and Y decreases.
- the concentration of both X and Y decreases with time.
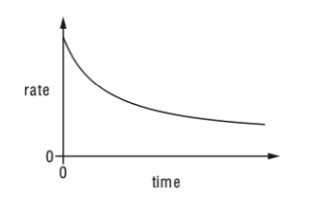
TBAT Chemistry-ข้อ 41
42. Hydrogen reacts with iodine to form hydrogen iodide.H2(g) + I2(g) -> 2HI(g)Which statements explain why the reaction is faster when the pressure is increased, at constant temperature ?
- At higher pressure, the molecules are moving faster.
- At higher pressure, more of the molecules have the required activation energy.
- At higher pressure, the molecules are closer together.
- At higher pressure, the molecules collide more frequently.
43. Ammonium sulfate is used as a fertiliser.It is made from ammonia and slfuric acid.The …1… is made by the …2… process in which …3… is used as a catalyst.Which words complete gaps 1, 2 and 3 ?
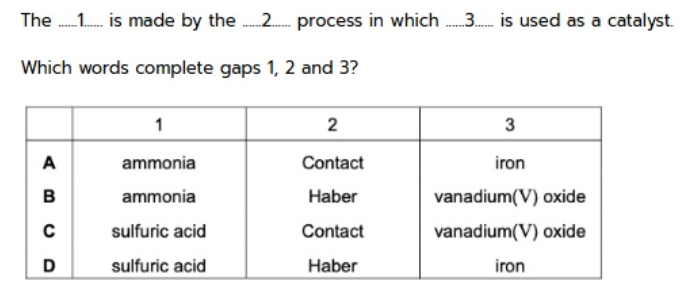
tbat chemistry ข้อ 43
44. The reversible reaction shown takes place in a closed system at constant temperature.P(g) + Q(g) + R(g) ⇌ S(g) + T(g)When the reaction has reached equilibrium, more T is added.After the addition of T, which other substances increase in concentration ?
45. In which equation is the underlined substance acting as a reducing agent ?
46. An aqueous solution reacts with a solid. The products are an alkaline gas, a salt and water.What are the aqueous solution and the solid ?
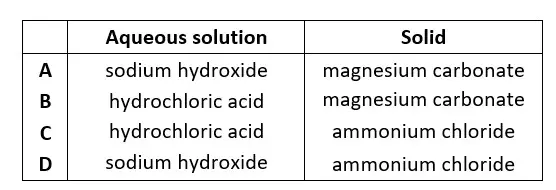
tbat chemistry q46
47. Butanoic acid partially dissociates in aqueous solution.Which row about butanoic acid is correct ?Copper(II) sulfate is prepared by adding excess copper(II) carbonate to sulfuric acid.Why is an excess of copper(II) carbonate added ?
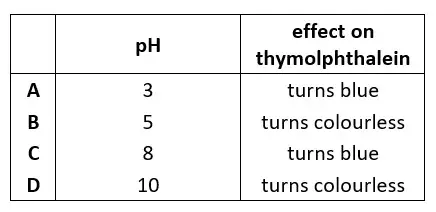
tbat chemistry q47
48. Part of the Periodic Table is shown.Which element has two electrons in its outer shell and three electron shells ?
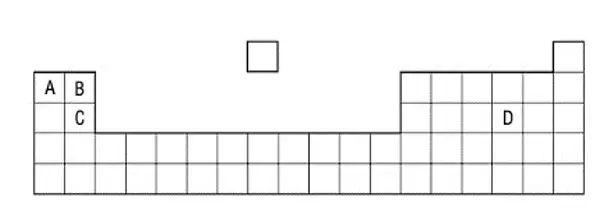
tbat chemistry q48
49. Elements in Group I and Group II show the same trends in their reactions with water and in their density.Which row shows how the properties of barium compare with calcium ?
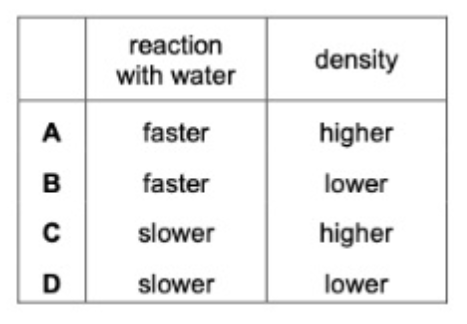
50. Which pair of compounds shows a transition element in two different oxidation states?
51. Which description of brass is correct ?
52. What is the symbol of the metal used in the manufacture of aircraft because of its low density ?
53. Which substances react to form hydrogen gas ?
- calcium and water
- silver and dilute hydrochloric acid
- magnesium and steam
- zinc and dilute hydrochloric acid
TBAT Chemistry
You got {{userScore}} out of {{maxScore}} correct
{{title}}
{{image}}
{{content}}
